Quality Assurance and Regulatory Affairs (QARA)
Global demand for medical and diagnostics devices is expected to be worth over USD 720 billion by 2029. With new and more stringent regulatory and quality compliance requirements shaping the global QARA landscape, are you, as a medical devices OEM, equipped to navigate the emerging dynamics?
LTTS leverages decades of deep domain experience, multi-industry engineering expertise, and a global delivery infrastructure to streamline your journey in the evolving medical device regulatory compliance ecosystem. We do more than just help your teams in regulatory submissions. Our global teams of experts and engineers align with your cross-functional units to evaluate risk, prioritize the most impactful products, and implement a business plan that makes sense for you.
We help you evaluate, integrate, and deliver across the various inter- and intra-geography medical regulations transforming and redefining the current paradigms in the process.

What We Offer
Regulatory Compliance
- Assessment for Country-specific Standards and Regulations
- Dossier/Technical Files for Regulatory Submissions
- New Market Registration
- Change Notifications
- Hazardous Substances Evaluation
- Clinical Evaluation
- Biocompatibility Evaluation

Product Quality Assurance
- Design Quality
- DHF/Techfile
- Functional Testing
- Reliability
- Pre-Compliance and Compliance Test

Quality Management System
- Gap Assessment
- Quality System Design and Deployment
- SaMD/SiMD Process
- QMS Harmonization and Integration
- Work Instructions – QMS tools and Processes

Process and Supplier Quality
- New Line Set-up
- Line Transfers
- Process Validations
- Computer System Validations
- TMV
- Supplier Qualifications and Audits
- Multi-organization Integration

Post Market Support
- Complaints Management
- CAPA
- Change Management
- Lifecycle Sustenance support
- PMS and Risk Management
- Remediations (e.g., MDD to EU MDR)

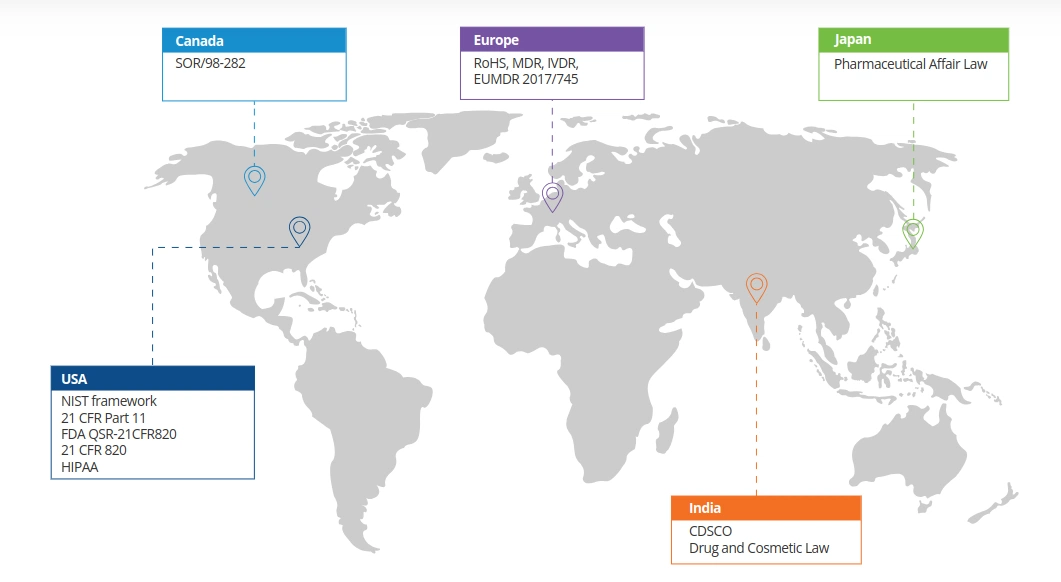
Regulations
LTTS Your Partner for Success

Who We Serve
LTTS’ robust reporting systems ensure full transparency and compliance with global regulatory standards. From detailed documentation to risk management and audit readiness, we help you navigate the complex regulatory landscape. Whether you are developing cutting-edge diagnostic tools or life-saving medical equipment, we ensure your products meet the highest quality standards, supporting your journey from concept to market with innovation, compliance, and excellence.
We proudly partner with medical device OEMs across the diagnostics, medical equipment, and life sciences sectors on a global scale.
Success Stories
What Stand Us Apart
Domain Experience and Expertise
A team of seasoned professionals with deep knowledge of global compliance. Extensive experience navigating FDA and EMA regulations for smooth market approvals.
State-of-the-Art Infrastructure
Innovative Methodologies and Tools, Customized, Value-Driven Solutions